
GAMP5 guidance aims to achieve computerized systems that are fit for intended use and meet current regulatory requirements, by building upon existing industry good practice in an efficient and effective manner.ĭue to the great variety of medical devices, processes, and manufacturing facilities, it is not possible to state in one document all of the specific validation elements that are applicable. GAMP has enjoyed the support of numerous regulatory authorities over the years spanning the United States, Europe, and Japan and is now a recognized good practice worldwide. Soon afterwards the organization entered into a partnership with ISPE, formally becoming part of ISPE in 2000. GAMP published its first guidance in 1994. Food and Drug Administration expectations for Good Manufacturing Practice (GMP) compliance of manufacturing and related systems. GAMP itself was founded in 1991 in the United Kingdom to deal with the evolving U.S. The purpose of the guidelines is to “provide a cost effective framework of good practice to ensure that computerized systems are fit for use and compliant with regulation.” These guidelines are the latest, up-to-date thinking in the approach to validation of GxP computerized systems. The new GAMP-5 guidelines were released February 2008 at the ISPE Manufacturing Excellence Conference in Tampa, Florida. The most well-known is The Good Automated Manufacturing Practice (GAMP) guide for Validation of Automated Systems in Pharmaceutical Manufacture.

#Gamp categories examples series
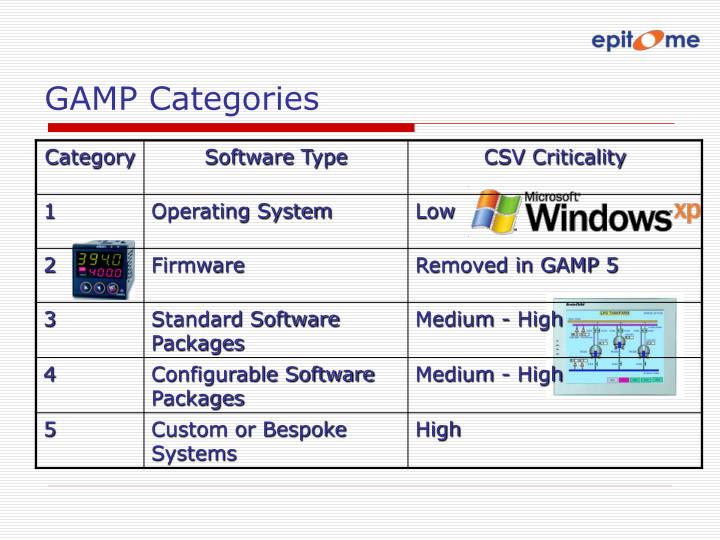
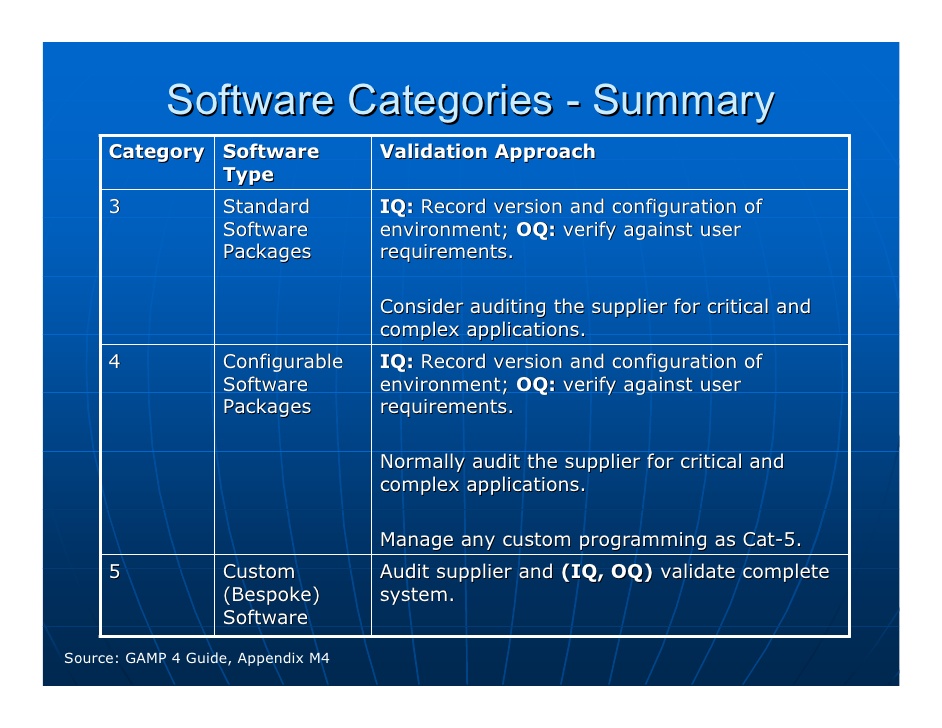
ISPE has published a series of good practice guides for the industry on several topics involved in drug manufacturing. Standard operating procedures (SOPs) are essential for processes that can affect the quality of the finished product. As a result, GAMP covers all aspects of production from the raw materials, facility and equipment to the training and hygiene of staff. Interior principles of GAMP is that quality cannot be tested into a batch of product but must be built into each stage of the manufacturing process. More specifically, the ISPE's guide Good Automated Manufacturing Practice (GAMP) guide for Validation of Automated Systems in Pharmaceutical Manufacture describes a set of principles and procedures that help ensure that pharmaceutical products have the required quality. Good Automated Manufacturing Practice (GAMP) is a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE), a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. Some applications of GAMP- 5 in Pharmaceutical industries like Monitoring manufacturing, production and storage environments in the pharmaceutical industry, Monitoring the autoclaving process in the pharmaceutical industry, Water purification in the pharmaceutical industry, Freeze drying in the pharmaceutical industryĬomputerized systems, Guidelines, Risk- based approach, Validation, Patient safety, Product quality. Understanding the product and process is critical in determining system requirements and for making science and risk-based decisions to ensure that the system is “fit for use.” In determining “fit for use,” attention should be focused on “those aspects that are critical to patient safety, product quality, and data integrity.” Defining a lifecycle approach to a computerized system has been expanded from GAMP 4 to include all phases and activities from concept and implementation through operation and retirement. Science Based Quality Risk Management.The purpose of the guidelines is to “provide a cost effective framework of good practice to ensure that computerized systems are fit for use and compliant with regulation.” There are five key concepts to GAMP 5:

The new Good automated manufacturing practices (GAMP)-5 guidelines were released February 2008 at the ISPE(International Society for Pharmaceutical Engineering) Manufacturing Excellence Conference in Tampa, Florida.


 0 kommentar(er)
0 kommentar(er)
